
The photocurrent density of the 2% Sn/ β-Fe 2O 3 photoanodes reaches 2.21 mA cm −2 at 1.6 V RHE, which is 8.5 times that of β-Fe 2O 3 photoanodes (Supplementary Fig. The as-prepared β-Fe 2O 3 photoanodes were tested for PEC simulated seawater splitting. These lattice atomic images and FFT patterns are completely consistent with the atomic arrangement of the corresponding crystal plane in the theoretical model. A clear atomic image of the (1 1 1) crystal plane taken from another region of β-Fe 2O 3 and fast Fourier transform (FFT) patterns of the (1 1 0) and (1 1 1) planes were obtained (Supplementary Fig. It was confirmed that the lattice position of Fe was substituted by Sn. One of the regions was selected for three-dimensional modelling, which shows the contrast difference between Sn atoms and surrounding Fe atoms (Fig. 1c correspond to the Sn single atom in the β-Fe 2O 3 lattice. Many bright spots with high contrast in the (1 1 0) crystal plane in Fig. A large area of lattice stripes indicates good crystallinity of β-Fe 2O 3 (Fig. The Sn/ β-Fe 2O 3 film is composed of blocks arranged vertically with a thickness of approximately 400 nm (Fig. Metastable β-Fe 2O 3 photoanodes doped with Sn were prepared by the spray pyrolysis method, and their phases were accurately determined (Supplementary Fig.

This study may ignite the dawn of application for PEC seawater splitting for hydrogen production and deepen the understanding of the seawater corrosion of oxides.
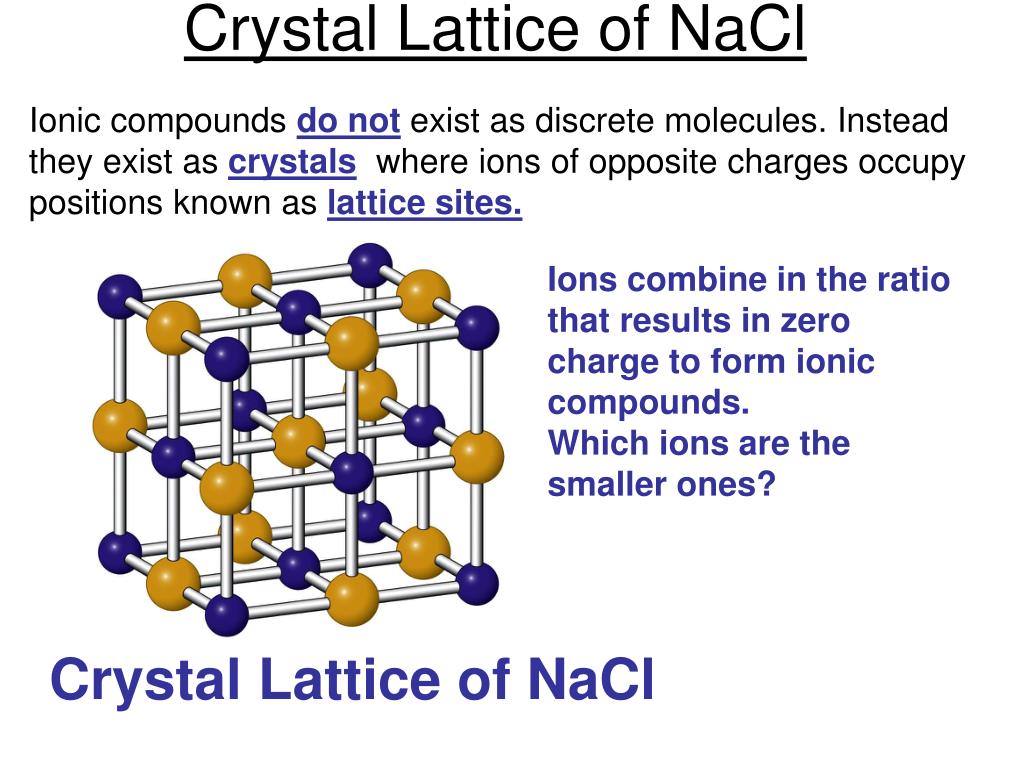
As a result, Sn/ β-Fe 2O 3 without any protective overlayer shows excellent durability in seawater splitting over 3000 h and is by far the most stable photoanode. Dispersed Sn single atoms in the lattice were found to endow the β-Fe 2O 3 photoanodes with good inhibition of hydration and resistance to Cl − attack in seawater. We have revealed that the Cl − ions in seawater will damage the surface hydrated layer of β-Fe 2O 3 photoanodes, thus remarkably reducing their stability. At the same time, β-Fe 2O 3 also shows good stability in photoelectrochemical alkaline water splitting 20, 21. Thus, it has a higher theoretical solar-to-hydrogen efficiency than α-Fe 2O 3. Because of its narrower band gap (1.9 eV) compared with α-Fe 2O 3 (2.1 eV), the theoretical optical absorption band edge can be extended to approximately 650 nm. Recently, β-Fe 2O 3 entered our research field as a metastable phase of iron oxide. Herein, we studied the effect of Cl − ions on the stability of a photoelectrode such as β-Fe 2O 3. Little attention has been given to improving the stability of photoelectrodes in aqueous electrolytes with Cl − ions 14, 15, since Cl − ions easily corrode photoelectrode materials and may participate in the competitive oxidation reaction to produce Cl 2 or ClO − 16, 17, 18, 19. Some strategies, such as protective layers, electrocatalysts, and tuning electrolyte composition, have been used to improve the durability of photoelectrodes in aqueous electrolytes without Cl − ions 11, 12, 13. However, except for iron oxide, almost all bare photoelectrodes show unsatisfactory stability in water splitting for hydrogen production, let alone in highly corrosive seawater 7, 8, 9, 10. The long-term stability of photoelectrodes is an essential prerequisite for the practical application of PEC water splitting for hydrogen production 6. The formation of an ionic compound is exothermic while the dissociation or the reverse of it is endothermic.The use of PEC water splitting to produce hydrogen can realize the conversion of solar energy to hydrogen energy in one step, which is a very promising solution for building a low-carbon society 1, 2, 3, 4, 5. While the distance between the charges of the ions or the ionic radii increases the lattice energy decreases. Hence, lattice energy associated with the crystal AB is 4X.Īs the charges on the ions, that is the cations and the anions, increase, the lattice energy of the compound also increases. Therefore, the lattice energy will be 4X. The formula of lattice energy can be derived as $ \right)$ Sodium chloride NaCl is an ionic compound that dissociates as: The ions thus formed are cation (positive ions) and anions (negative ions). Lattice energy can be defined as the energy required to completely separate the ions of an ionic compound into their gas states. It is derived by the charges on both ions upon the distance between the two charged atoms in the ionic compound. Hint: lattice energy of any compound is the energy that is required to completely separate the respective ions in an ionic compound that form their respective gaseous states.


 0 kommentar(er)
0 kommentar(er)
